In medical device manufacturing, a medical device’s label must clearly display the necessary information to ensure not only the safety of the end user but also legal compliance. This includes a series of key signs and symbols, which should be clearly displayed in a certain way.
Medical device labelling requirements can vary according to the market in which the device is to be used or the specific product details – and labelling must adhere to those exact requirements.
Generally, information given on medical device labels will include:
- Details of what the product is
- The address of manufacturer
- Company logo
- Contact details
- Reorder Number (REF)
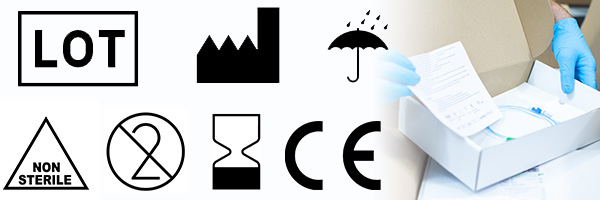
- LOT number
- Date of manufacture
- Expiry date
- Method of sterilisation (where applicable)
- Consult instructions for use
- Unique device identifier (UDI)
- CE number (where applicable)
Additional information on performance testing and tolerances, storage instructions and details of certain ingredients, for example, may also be required.
Key signs and symbols on medical device labelling
Some of the key signs and symbols that you are likely to see on medical devices include the following:
CE MARKING

The CE mark indicates conformity with EU health, safety, and environmental protection standards on many products that are traded on the single market in the European Economic Area (EEA). Where used, it is vital that the initials ‘CE’ are printed in the standard, recognisable form and adhere to the following rules:
- if you reduce or enlarge the size of your marking the letters CE must be in proportion to the standard version
- the CE marking is at least 5 millimetres – unless a larger minimum dimension is specified in the relevant directive
- the CE marking is easily visible, readable and permanent
Other commonly used key signs and symbols on medical device labelling, include the following:
 |
ATTENTION This indicates that attention must be given before using the product. For example, it may lead on to guidance that instructions are included in the packaging and must be read prior to use. In this case the attention symbol will be accompanied by a symbol of a book. |
 |
KEEP DRY Indicates a medical device that needs to be protected from moisture. |
 |
Product Expiry Date / Use By – this symbol will be next to the date (year/ month) on which a product must be used by. |
 |
The white factory indicates the date that the product was manufactured. |
 |
The black factory shows details of the product manufacturer and gives that address. It is important to note the difference between the two. |
 |
Single use symbol, which means that the medical device must only be used once and then disposed of in the correct manner. |
 |
The product must not be re sterilised. |
 |
This symbol indicates that the medical device is sterile, along with the method by which it has been sterilised – in this specific case ethylene oxide. Other medical product sterilisation methods can include radiation (R), x-ray (E) and hydrocholric acid (H). Proper packaging, such as using medical pouch packaging, is essential to maintain sterility until the point of use. |
 |
The product has not been sterilised. |
 |
Do not use the product if the box is damaged. |
 |
The manufacturing LOT number of the product is displayed next to this symbol. This ensures traceability in the manufacturing process. |
 |
Reference number of the device. The reorder code is contained next to this symbol for those wishing to order more. |
Medical devices for a range of uses
Meridian Medical is a UK-based contract medical equipment manufacturer, specialising in a wide range of medical device design, development and supply services.
To find out more about how we can help you with your medical product design and manufacture, get in touch today by filling out our online form or contact us on 01903 732344 or info@meridian-medical.com.

Author: Andrew Wootten, Quality and Regulatory Manager
Andrew Wootten has been at the forefront of Meridian Medical’s quality and regulatory functions for over a decade. With a career in quality management that began in 1989 and extensive experience in the medical device sector since 2002, Andrew is a seasoned expert. He holds a City and Guilds certificate in Quality Assurance, a diploma in Quality Management from BSI, and is a certified internal auditor for ISO 13485, ISO 9001, and ISO 14001. Andrew ensures our regulatory compliance and drives the company’s commitment to excellence in quality and safety, making him a trusted authority in the industry. His deep understanding of global regulatory challenges and unwavering dedication to quality assurance position him as a key figure in delivering safe, compliant and top-quality medical devices.




