In the highly regulated world of medical device manufacturing, ensuring that each aspect of your production consistently meets specification is critical. A robust Medical Device Validation Plan plays a vital role in this process. This blog will...
Andrew Wotten
FREE Medical Device Supplier Audit Checklist
A practical, quick reference-style audit checklist A free Audit checklist to help you audit your suppliers As a medical devices assembly expert, and having worked with many medical device companies, quality assurance professionals and notified...
How are medical devices approved in the UK?
When launching a new medical device or updating the specification to an existing product, your company needs to ensure that it has the correct regulatory approvals before releasing the device for human use or general sale. In the UK, medical...
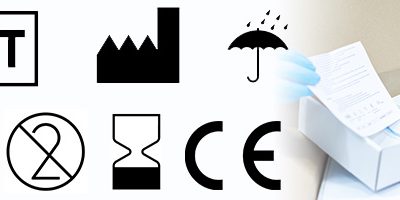
12 key signs and symbols on medical devices and packaging
In medical device manufacturing, a medical device’s label must clearly display the necessary information to ensure not only the safety of the end user but also legal compliance. This includes a series of key signs and symbols, which should be...
Medical device labelling – how to get it right
It is critical that you get medical device labelling right. If your product is used improperly as a result of incorrect or insufficient labelling, you can be hit with significant fines, costly delays and reputational damage. In medical device...
Are you ready for the new Medical Device Regulation?
The new European Medical Device Regulation (MDR) came into force on 26th May, 2020, bringing with it the most radical overhaul of the medical device regulatory framework that the medical device industry had seen in decades. The primary objective of...
The importance of medical device labelling
Medical device labelling is a critical part of the medical device manufacturing process. Accurate labelling of medical devices is vital to ensuring market access, and the safe and proper use of medical devices by patients and care givers. Labelling...
The importance of cleanrooms to disposable medical device manufacture
The operation of advanced cleanroom facilities is a crucial component of the medical device assembly process and has a critical role in ensuring the quality, safety and success of medical devices. Click the link to find out what a cleanroom is. If...
Quality Assurance in disposable medical devices
Quality Assurance should not be a unique selling point of a company involved in the manufacture and supply of disposable medical devices. A standard requirement of a company should be that the smallest detail matters. Everything should be perfect....