When launching a new medical device or updating the specification to an existing product, your company needs to ensure that it has the correct regulatory approvals before releasing the device for human use or general sale.
In the UK, medical devices are regulated by the Medicines and Healthcare products Regulatory Agency (MHRA), which oversees the approval process to ensure that devices are safe, effective and meet the essential requirements of relevant legislation. In this blog, we will delve into the approval process for medical devices in the UK, including the requirements for CE marking and meeting the Medical Device Regulation (MDR) and give a brief overview of the certification process.
The approval process for medical devices
Before submitting an application, you should have already registered your company with a notified body. Once you have this certification completed, you can then register your device or range of devices.
The approval process for medical devices in the UK involves several steps: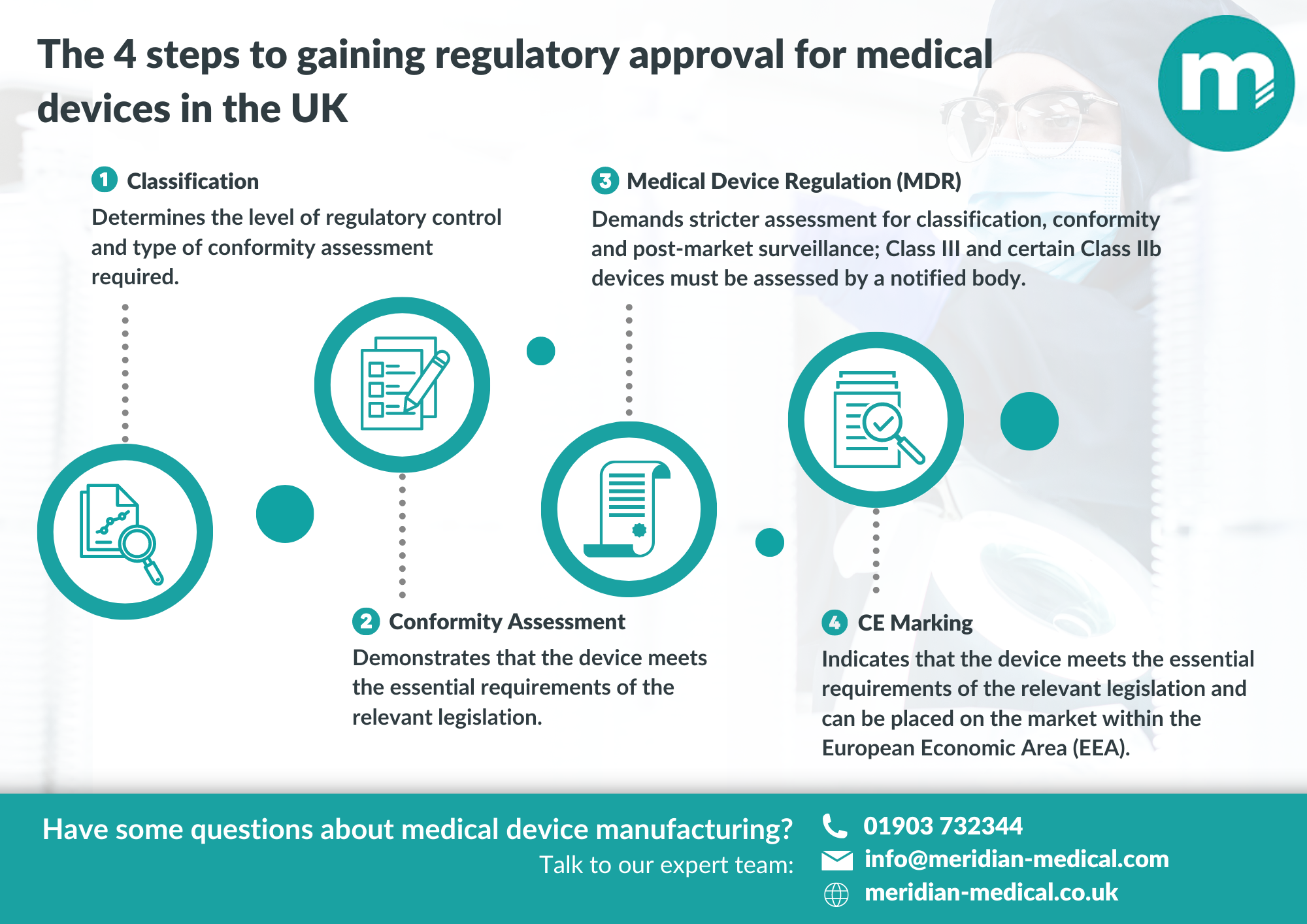
- Classification: Medical devices are categorised into four classes based on their risk level. Class I devices are considered low risk, while Class III devices are high risk. The classification of a device determines the level of regulatory control and the type of conformity assessment required.
- Conformity Assessment: Once a device has been classified, the manufacturer must carry out a conformity assessment to demonstrate that the device meets the essential requirements of the relevant legislation. There are several routes to conformity assessment, including self-certification, third-party assessment and full quality assurance. The type of assessment required depends on the classification of the device.
- CE Marking: After completing the conformity assessment, the manufacturer must apply for CE marking, which indicates that the device meets the essential requirements of the relevant legislation and can be placed on the market within the European Economic Area (EEA). The CE marking is a legal requirement and must be affixed to the device or its packaging. It is important to note that CE marking is not a guarantee of quality or safety; it simply indicates that the device meets the essential requirements of the legislation.
- Meeting MDR: The Medical Device Regulation (MDR) came into effect in May 2021, replacing the previous Medical Devices Directive (MDD). The MDR introduced new requirements for the approval of medical devices, including stricter rules for classification, conformity assessment and post-market surveillance. Under the MDR, Class III devices and certain Class IIb devices must be assessed by a notified body. Notified bodies are independent organisations designated by the MHRA to assess the conformity of medical devices with the essential requirements of the legislation.
In addition to the requirements for classification and conformity assessment, the MDR also introduced new requirements for post-market surveillance and clinical evidence. Manufacturers must monitor their devices once they are on the market and report any adverse events to the relevant authorities. They must also gather clinical evidence to support the safety and effectiveness of their devices.
In addition to these steps, medical devices may also require approval from other regulatory bodies, such as the National Institute for Health and Care Excellence (NICE) for reimbursement and the Health and Safety Executive (HSE) for workplace safety.
Patient safety and device effectiveness
The approval process for medical devices in the UK is a complex and rigorous process that is designed to ensure patient safety and device effectiveness. Manufacturers must follow a series of steps, including classification, conformity assessment and CE marking, to demonstrate that their devices meet the essential requirements of the relevant legislation. The introduction of the MDR has added new requirements for manufacturers, including stricter rules for classification, conformity assessment, and post-market surveillance. By following these requirements, manufacturers can ensure that their devices are safe, effective, and meet the highest standards of quality.
Meridian Medical is an established and experienced contract medical device manufacturer specialising in a wide range of medical devices. We offer design, regulatory assistance, cleanroom injection moulding, cleanroom assembly, contact packaging and sterilisation management. We have over 30 years’ experience supplying UK and European companies.
To find out more about how Meridian Medical can help you with your medical device manufacture, get in touch today by filling out our online form or contacting us on 01903 732344 or info@meridian-medical.com.

Author Andrew Wotten
Andrew Wootten has been at the forefront of Meridian Medical’s quality and regulatory functions for over a decade. With a career in quality management that began in 1989 and extensive experience in the medical device sector since 2002, Andrew is a seasoned expert. He holds a City and Guilds certificate in Quality Assurance, a diploma in Quality Management from BSI, and is a certified internal auditor for ISO 13485, ISO 9001, and ISO 14001. Andrew ensures regulatory compliance and drives the company’s commitment to excellence in quality and safety, making him a trusted authority in the industry. His deep understanding of global regulatory challenges and unwavering dedication to quality assurance position him as a key figure in delivering safe, compliant, and top-quality medical devices.