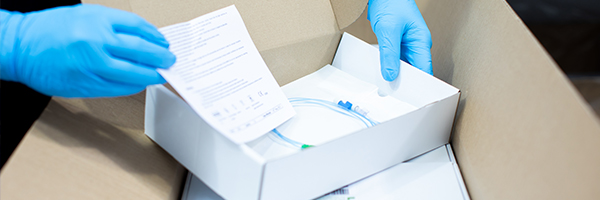
It may ultimately be destined for the bin but medical device packaging is an important aspect of a product’s design and success. As such a vital part of the product development process, any mistakes with the packaging of a medical device could cause costly delays, recalls and reputational damage.
When a sterile medical device arrives at a hospital or clinic, its packaging must not have any holes or tears in it and its seal must be intact. In short, it must be undamaged. The device has to be able to sit on a shelf, possibly for years, and its packaging remain in this as-new condition.
How to get your medical device packaging right
To help you get medical device packaging right, and avoid the risks associated with product damage, the poor performance of a product or the improper use of a product, here are nine key points to consider when planning your medical product packaging.
- Prioritise packaging. You should start the development of your medical device packaging at an early stage. This gives you invaluable time and flexibility to make changes as required. Leave it too late to organise packaging and addressing any issues could lead to costly delays.
- Type of packaging. There are a number of medical device packaging solutions available, including medical pouch packaging such as peel pouches and sachets, barrier packaging, thermoformed blisters and lids, and procedure packs. Peel packaging is one of the most commonly used forms of medical device packaging.
- Packaging size. When choosing your packaging, it is important to use a product prototype that has the same dimensions as the finished product. Any significant differences in size could compromise the product and delay its launch or distribution.
- Sterilisation requirements. It is important that you are clear about your sterilisation requirements because these will influence the sterilisation method of your medical device and, in turn, the type of packaging you need.
- Packaging shelf life. If your medical device is likely to sit on a shelf for an extended period, your packaging must be able to withstand prolonged storage and the conditions related to its storage.
- User interaction and experience. A lot of people come into contact with a medical device during its lifespan, from doctors, nurses and other healthcare professionals to various personnel along the design, distribution and delivery chains. You should design your packaging with this use in mind.
- Regulatory compliance. Medical device packaging regulations can vary according to the market and your packaging must adhere to the appropriate standards to get market access. For example, packaging regulations in the US differ from those applied in the European Union. Furthermore, it is worth considering that regulations in the UK may soon differ from those in the European Union, with the introduction of the European Medical Device Regulation (MDR) in May 2020.
- Transit validation. It is essential that your medical device packaging is able to withstand the stresses of transportation if you are going to ship your products around the world.
- Packaging costs. The cost of your packaging should be appropriate to the value of your medical device. For example, low-cost devices are likely to require simpler packaging solutions, while higher-cost devices may need more sophisticated packaging.
Medical device packaging and your product packaging partner
Getting medical device packaging right can be a complex process. Assistance with packaging is one of the benefits of working with an expert contract manufacturer of medical devices.
The right medical device manufacturing partner will provide specialist advice on medical device packaging, guiding you through the process and ensuring that you have the packaging that best suits your needs and that you start developing it at the right time. They will also work with you to ensure that your packaging complies with all the relevant standards.
If your medical device contract manufacturer doesn’t offer these medical product packaging services, it’s time to reconsider your choice of partner.
Meridian Medical is an established and experienced contract medical equipment manufacturer, specialising in a wide range of medical device design, development and supply services.
To find out more about how Meridian Medical can help you with your medical product design and manufacture, get in touch today by filling out our online form or contacting us on 01903 732344 or info@meridian-medical.com.

Author: Paul Kearsley,
Technical Manager, Meridian Medical
Paul Kearsley brings a lifetime of engineering expertise to his role as Technical Manager at Meridian Medical. With an HNC in Mechanical Engineering and a foundation built during a four-year engineering apprenticeship, Paul has spent over 40 years running his own design company. Over the decades, he has designed more than 1,000 consumer, industrial, and medical devices, showcasing his ability to deliver innovative solutions across a broad spectrum of industries.
A pioneer in the transition from traditional drafting techniques to modern CAD workflows, Paul began his career on the drawing board, adopted AutoCAD 2D early on, and embraced 3D modelling with SolidWorks in 1997. His vast experience and technical leadership play a key role in Meridian Medical’s commitment to producing high-quality, single-use medical devices.



